Kuniaki ARA, Japan Atomic Energy Agency
Classification
5 - A
Liquid sodium is used for coolant of the fast reactor, because of its superior thermal fluidity and good compatibility with structural materials. However there is a weak point that is high chemical reactivity with oxygen or water. In this study, we are developing the reaction suppression technology of liquid sodium itself using atomic interaction between sodium atoms and nanoparticle which is suspended in liquid sodium. From the results, compared to liquid sodium, the suppression of sodium suspended nanoparticles of combustion reaction and water reaction confirms and their mechanisms became clear from the viewpoints of theoretically and experimentally. Applying this new technology of sodium suspended nanoparticles, not only the safety enhancement of fast reactor, but also there is a possibility of creation of new design concepts of fast reactor.
- (1) Components: container facility
- (2) Location:
sodium pipe, steam generator
- (3) Materials: liquid sodium
- (4) Condition: sodium leak fire and sodium water reaction under operation
(1) Characteristics of sodium
Sodium is solid at room temperature and it has metallic gloss in inert gas. Melting temperature is 98 deg.C, boiling point is 883 deg.C, and a temperature range of liquid state is very wide. Although the specific gravity depends on temperature, it is 0.97 at room temperature and it is lighter slightly than water. Sodium is extremely high reactivity metal and it oxides easily in air. It reacts with water intensely, and it occurs high reaction heat and hydrogen. Electronegativity of sodium is small than that of transition metals and it emits electron and exists stably with cation. Therefore the concentration of oxygen in liquid sodium is controlled lower than 10ppm in fast reactor, liquid sodium has the excellent compatibility with structural materials such as pipes and equipment. Common uses of sodium irrespective of coolant for fast reactor are a coolant of engine valve for vehicle and cathode of NaS battery utilizing its high thermal conductivity.
(2) Sodium Nanofluid
(2-1) Idea of reaction suppression
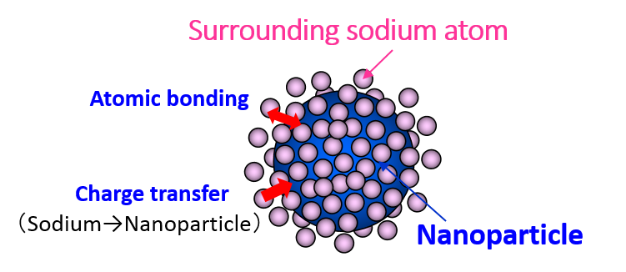
Idea of reaction suppression of liquid sodium itself is to suspend the metallic nanoparticles in liquid sodium. An image of suspension state of nanoparticle in liquid sodium is shown in Fig.1. Metal atoms on surface of nanoparticle interact stronger with the surrounding sodium atoms. It is an image that nanoparticle catches many sodium atoms. When sodium atom reacts with water or oxygen, sodium atom reacts after cutting atomic bonding between sodium atoms. On the other hand, when sodium nanofluid which has stronger the atomic bonding between nanoparticle and sodium atoms reacts with water or oxygen, sodium atom has to cut the stronger atomic bonding with nanoparticle. Therefore it required the extra-energy and extra-time compared with all the sodium atoms hence the reaction heat and the reaction rate are suppressed. On the basis of this
simple idea, there is no study of chemical reactivity suppression of liquid sodium by suspending nanoparticles. This idea was created uniquely by authors [1.2].
Fig.1 Image of suspended nanoparticle in liquid sodium.
Although the solubility of transition metals in liquid sodium is extremely low, metallic atoms exceeding the solubility limit are dispersed as nanoparticles and are technically stable. This is a new liquid metal of solid-liquid system. However this is non-equivalent system theoretically, a new solid-liquid system is stably in in actuality. In order to establish this solid-liquid system, there are some necessary requirements which are fine particle, low gravity for no settling, good wettability of surface of nanoparticle with liquid sodium and single-particle suspension. In this study the atomic interaction between surface atom of nanoparticle and sodium atom is used for the suspension of chemical reactivity of liquid sodium [4,5,6]. The amount of reaction suppression is proportional to breadth of contact area between nanoparticle and sodium atom. The smaller nanoparticle, the larger contact surface. Therefore in order to increase the specific surface area of nanoparticle, it is necessary the nanoparticle of smaller diameter. The further of this proposed idea by authors is to obtain the beneficial reactivity suppression by using the small amount of nanoparticle with small diameter.
For this reason the original superior thermal fluidity of liquid sodium is kept and it is possible to suppress only the chemical reactivity of liquid sodium. There are many studies of enhancement of property of solvent by suspending nanoparticles [7,8,9]. In particular, there are some studies of an improvement of thermal conductivity of water by suspension of metallic or ceramics nanoparticles. However, there have been no study using the atomic interaction by suspending nanoparticles in liquid sodium as solvent. Recently, Korea and France are interested in this idea, they started such studies [10,11].
(2-2) Research approach
This study is the fusion research of sodium technology and nanotechnology which is using the atomic interaction in range of atom and electron. For that reason we have been progressed this study by combination of both theoretical calculations and experiments, and by supplementing them each other. The research approach of this study is shown as follows.
- Estimation of the atomic interaction between surface atom on nanoparticle and sodium atom by theoretical quantum chemistry calculation.
- Fabrication of sodium nanofluid and verification of estimated atomic interaction by physical property evaluation.
- Evaluation of suppression effect of reaction properties with water or oxygen using the produced nanoparticle.
- Prediction in practical use of sodium nanofluid using obtained reaction suppression effect.
(2-3) Atomic interaction between nanoparticle and sodium
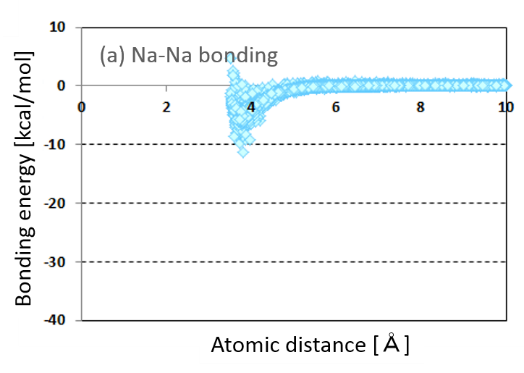
The atomic interaction between the surface atoms on nanoparticle and the surrounding sodium atoms occurs by suspension of nanoparticles in liquid sodium. Therefor in order to verify the atomic interaction, an electronic state of nanoparticle in liquid sodium was calculated using the multi-scale multi-physics chemical calculation on the basis of the Ultra-Accelerated Quantum Chemical Molecular Dynamics. The feature of this calculation is very high speed and it is possible to calculate in liquid state. We focused on atomic bonding and charge transfer as the atomic interaction. Calculated atomic bonding is shown in Fig.2(a) and (b). The atomic bonding between sodium atoms is shown in Fig.2(a). By contrast the atomic bonding between on nanoparticle and sodium atom is shown in Fig.2(b). The atomic bonding between nanoparticle and sodium is stronger than that between sodium atoms. As explained in the idea of sodium nanofluid, it became clear theoretically the nanoparticle bonds stronger with sodium atoms. Its strong atomic bonding between nanoparticle and sodium atoms suggests the stable suspension liquid sodium and no occurrence of reagglomeration.
Fig.2 (a) Atomic bonding between sodium atoms.
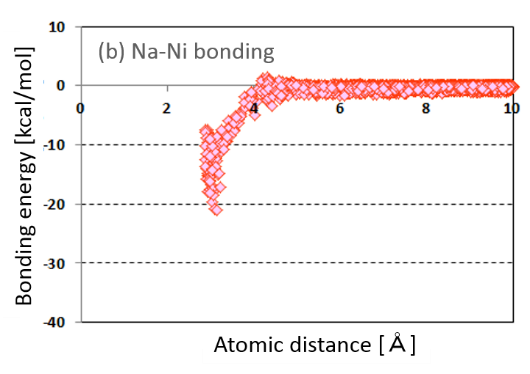
Fig.2 (b) Atomic bonding between on nanoparticle and sodium atom.
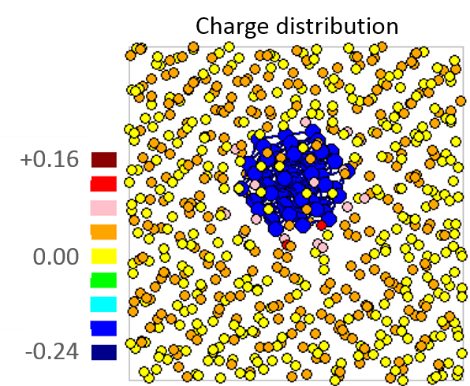
Fig.3 Charge transfer from surrounding sodium atoms to nickel nanoparticle.
Charge transfer occurred to nanoparticle from the surrounding sodium atoms. It depends on electronegativity. Amount of charge transfer to nanoparticle from surrounding sodium atoms was very slightly. From these results, it became clear the electronic state of surrounding nanoparticle such as atomic bonding and charge transfer at first when the nanoparticles suspended in liquid sodium.
(3) Reactivity suppression by atomic interaction
(3-1) Reactivity suppression by atomic bonding
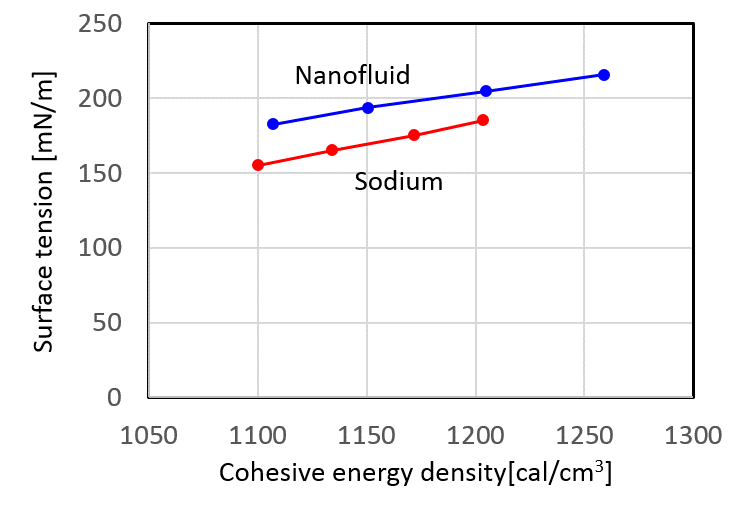
It became clear that the atomic bonding was stronger by suspension of nanoparticles in liquid sodium. So the cohesive energy of sodium nanofluid was investigated. Cohesive energy density indicates the required energy in order to detach the bonding atoms to each single atom. Hence, a larger value means a strong bonding of nanofuid. From these results, the atomic bonding becomes stronger than sodium by suspending nanoparticles and it is expected that physical property related by the cohesive energy changes. It is predicted that the physical properties of sodium nanofluid change with changing the atomic interaction by suspension of nanoparticles. The estimated atomic interaction was verified by the change of physical properties of nanofluid. Surface tension and evaporation rate which relate to the cohesive energy density were measured and these changes of sodium nanofluid were compared and confirmed with these of sodium [16]. Depending on the cohesive energy, the surface tension of sodium nanofluid was larger than that of sodium (Fig.4). It results from that the atomic interaction between nanoparticle and sodium increase. And the evaporation rate of sodium nanofluid decreased lower than that of sodium. It also results from the cohesive energy density increased. From these results the theoretical atomic interaction of sodium nanofluid was verified by these physical properties obtained by experiments. It is expected that chemical reactivity suppression due to the change of these physical properties.
(3-2) Reactivity suppression by charge transfer
A reaction profile of sodium water reaction was already calculated theoretically [15]. Chemical reaction occurs a giving and receiving of electron. It is expected that an energy state in reaction process changes by the charge transfer from sodium atoms to nanoparticle. The reaction profile when the charge transfer occurred was calculated. From this result, the energy of transient state and the activation energy of primary reaction and secondary reaction increased and reaction rate became slow. Moreover the energy state of an initial state before reaction became stably, enthalpy was decreased. The decrease of enthalpy results from reduction of reaction heat.
Fig.4 Relationship of surface tension between nanofluid and sodium.
(1) Reaction suppression effect and its mechanism
(1-1) Combustion reaction
(1-1-1) Combustion behavior of sodium
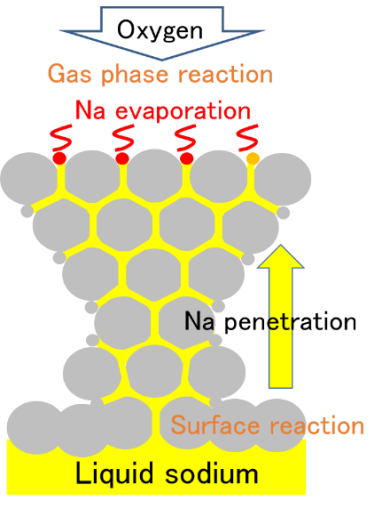
In general, there are two types of combustion behavior of material. One is gas phase reaction between evaporated flammable vapor such as hydrocarbon and oxygen. Another is surface reaction between carbon on surface and oxygen. In this section, the feature of sodium as alkali metal described compared to general materials. Surface reaction of sodium combustion is mainly at first stage. Rising of sodium temperature by surface reaction, sodium vapor occurs from the surface and the surface reaction transfer to the gas phase reaction. The feature of sodium combustion is transition from the surface reaction to the gas phase reaction. Morphology of oxidation reaction of sodium is shown in Fig.5(a). Dendritic oxidation of surface is grown. Schematic draw of growing up of oxidation is shown in Fig.5(b). Oxide grows up like dendrite and liquid sodium supply to top of dendrite from the bottom. The gas phase reaction occurs by the evaporated sodium on the top. This reaction behavior is explained in detail below.
Fig.5 (a) Combustion behavior of liquid sodium. (Movie)
- Leaked sodium from pipe accumulates on the liner and oxidation on surface occurs accompanied by reaction heat. Mixed sodium oxide having high melting temperature (1132 deg.C) and sodium peroxide having low melting temperature (675 deg.C) are formed as the oxide layer.
- Sodium temperature rises by reaction heat and both temperature of atmosphere and structure rises in the same way.
- For sodium peroxide exceeds its melting temperature due to temperature rise, it melts to liquid sodium including oxygen. This liquid sodium becomes supply path of oxygen to reaction surface and sodium combustion continues.
- Exudated liquid sodium to reaction surface evaporates and reacts with oxygen, then sodium oxide and sodium peroxide are formed accompanied by reaction heat. This phenomenon repeats many times and liquid sodium penetrates into oxide layer. Oxide layer grows while evaporating and oxide reaction proceeds.
Fig.5 (b) Schematic diagram of liquid sodium combustion.
(1-1-2) Combustion behavior of sodium nanofluid
(1-1-2-1) Suppression of thermal damage
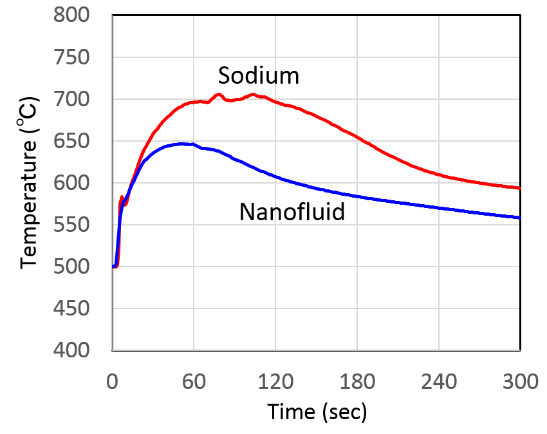
Combustion reaction of sodium nanofluid is suppressed by decrease of evaporation rate and checking reaction heat of sodium nanofluid. Therefore, the combustion experiments of sodium nanofluid and sodium were carried out to investigate the suppression effect by temperature in the course of combustion. Sodium nanofluid and sodium were put on combustion plates in argon atmosphere and they heated to 500 deg.C. Air (oxygen concentration 20%) is blown from above at same time, the combustion behavior of sodium nanofluid and sodium were observed. Because it is important to keep as lower as possible, the temperature of floor liner steel, considering thermal damage to structure by sodium leak accident at plant, temperature of lower part of combustion plate was evaluated with changing combustion time.
Fig.6 Combustion temperature profile of nanofluid and sodium.
Temperature changes corresponding to that of floor liner with time are shown in Fig.6. From these results, temperature of sodium nanofluid was lower than that of sodium. It is considered that it is due to the decrease of evaporation rate by atomic bonding and the reduction of reaction heat by charge transfer. It became clear that combustion is suppressed by the atomic interaction. Also it was confirmed the combustion rate of sodium nanofluid decreased compared to that of sodium from a reduction rate of diameter of droplet on combustion behavior of static droplet experiment. It is expected that this suppression behavior is due to the decrease of evaporation rate by atomic bonding.
(1-1-2-2) Self-termination of combustion
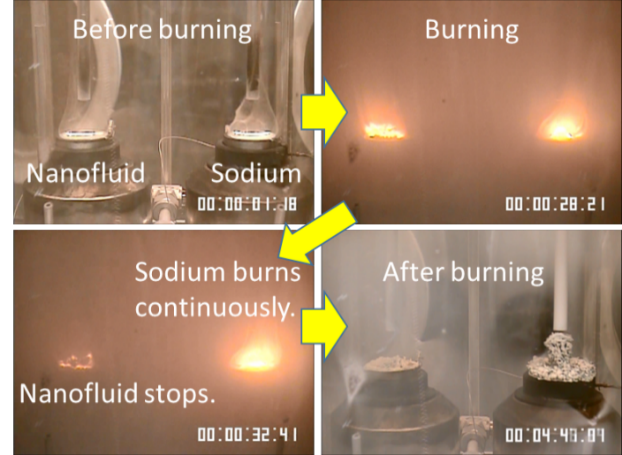
Self-termination of combustion was observed as the specific suppression feature of sodium nanofluid. We described sodium combustion behavior in 5.(1-.1-)1 section, here that of sodium nanofluid was shown in Fig.7 compared to that of sodium. Left side is sodium nanofluid and right side is sodium in each figure. The combustion behavior of sodium nanofluid and sodium was observed at 500deg.C. From this result, an ignition occurs at the same time, but the combustion of sodium nanofluid stopped in the middle, though that of sodium continued until sodium disappearance. We call it self-termination of combustion. Only the surface part of sodium nanofluid combusted, and non-react sodium nanofluid was remained under surface. On the contrary, all sodium combusted and non-reacted sodium was not observed.
Fig.7 Comparison of combustion behavior between nanofluid and sodium. (Movie)
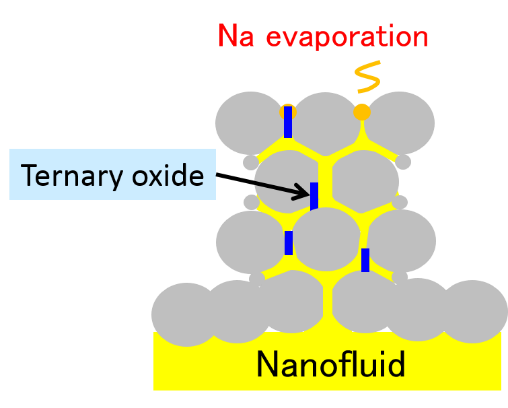
This self-termination mechanism is considered the following as shown in Fig.8(a) and (b). Sodium combustion form the mix oxide of sodium oxide and sodium peroxide. Rising temperature of oxidation by combustion, sodium peroxide is dissolved and liquid sodium is supplied to top of oxides. Liquid sodium evaporates and sodium vapor combust continuously.
On the contrary, many stable ternary oxides including the element of nanoparticle are formed in oxide layer as shown in Fig.8(a). Formed oxides have high melting temperature and stable thermodynamically. Hence, they are not dissolved even at high temperature. Hence the supplying path of liquid sodium in oxide layer is cut off and combusting sodium is not supplied to top of oxides and the combustion of sodium nanofluid terminates. The terminating behavior of growing oxides of sodium nanofluid is shown in Fig.8(b).
Fig.8 (a) Schematic diagram of nanofluid combustion.
Fig.8 (b) Combustion behavior of nanofluid. (Movie)
This self-termination mechanism is due to form the stable ionic oxide with nanoparticle, sodium and oxygen. From this effect, the combustion time of sodium nanofluid becomes shorter than that of sodium and the thermal damages to structure and atmosphere reduce. Especially the thermal damage of floor liner reduces and mechanical property of floor liner is kept by the suppression of rising temperature.
(1-2) Sodium-water reaction
(1-2-1) Sodium-water reaction behavior of sodium
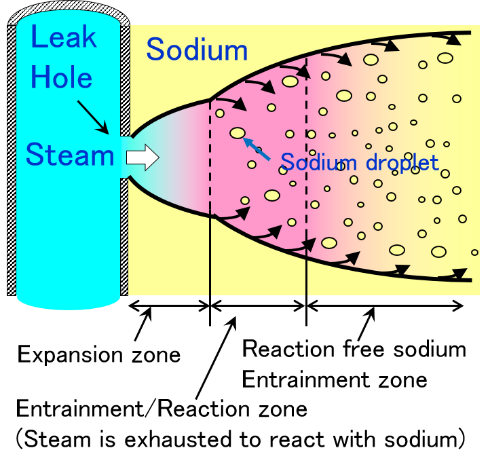
Sodium-water reaction occurs by the damage of heat transfer tube in steam generator. High pressure steam is filled in thermal transfer tube and there is liquid sodium in outside of heat transfer tube. Sodium-water reaction behavior occurs by ejecting high temperature and high pressure vapor into liquid sodium. This phenomenon is shown in Fig.9(a) and reaction process of sodium-water reaction is describes as follows.
Fig.9 (a) Behavior of sodium-water reaction.
Fig.9 (b) Reaction of entrained sodium droplet.
- High temperature and high pressure vapor eject from tube and expands adiabatically.
- After that an entrainment of liquid sodium into vapor from surrounding liquid sodium occurs.
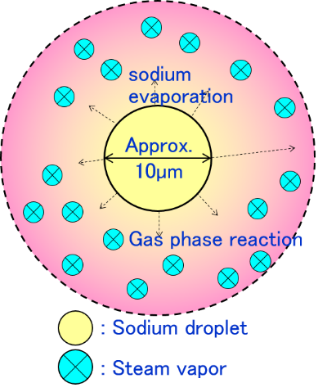
- Entrained liquid sodium is formed a droplet having about 10 micro-meter diameter by surface tension.
- Sodium evaporates from the droplet entrained into high temperature vapor and it reacts with the surrounding water vapor and generates the reaction heat in a moment (Fig.9(b)). The reaction between sodium vapor and water vapor is significantly fast and large amount of reaction heat.
- Reaction jet is formed by jet behavior with reaction. And sodium temperature rises higher and sodium hydroxide and hydrogen was created by reaction.
Thermal transfer tubes in steam generator near the damaged tube occur by the high temperature rupture and damage of wastage. The rupture of tube is propagated in the surrounding tubes. Furthermore temperature of sodium becomes higher and large amount of hydrogen generates.
(2-1) Sodium-water reaction behavior of sodium nanofluid
Here, the reaction suppression effect of sodium nanofluid on sodium-water reaction is described as follows. As explained earlier, the entrained sodium into steam forms the droplet and sodium vapor from the surface of the droplet react the surrounding vapor. The size of entrained droplet of sodium nanofluid is larger than that of sodium, because the surface tension of sodium nanofluid is larger than that of sodium. From this reason the area of evaporation surface decrease. Also, for the evaporation rate of sodium nanofluid reduces by atomic bonding, amount of sodium vapor which react with water vapor decrease. In addition, the reaction heat of sodium nanofluid with water becomes smaller than that of sodium due to the charge transfer to nanoparticle from sodium atoms. Temperature of reaction jet reduces by reaction suppression effect [18].
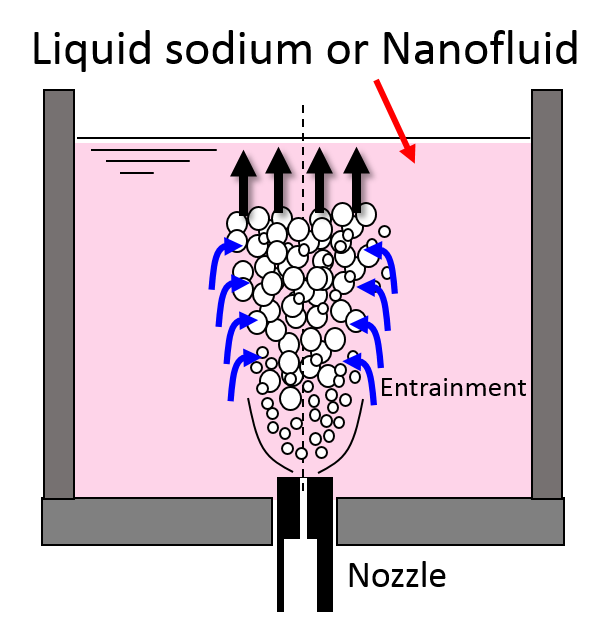
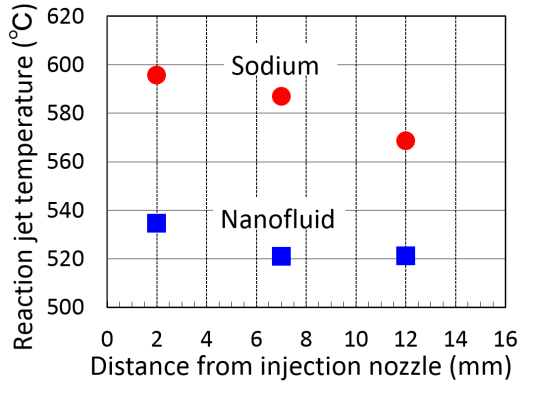
Sodium nanofuid and sodium with water reaction experiments were carried out as shown in Fig.10(a). From a result, reaction temperature of sodium nanofluid surely decreased than that of sodium as shown in Fig.10(b). The above speculation of the reaction suppression was verified by these experimental results. Furthermore it is expected that an area of high temperature of reaction jet reduces.
Fig.10 (a) Schematic diagram of sodium-water reaction experimental apparatus.
Fig.10 (b) Comparison of reaction jet temperature between sodium nanofluid and sodium.
(3) Applicability of sodium nanofluid to fast reactor
(3-1) Applicability as coolant
The purpose of sodium nanofluid is to reduce the impact to plant at the accident by the chemical
Table 1 Investigation items of applicability assessment of sodium nanofluid to practical plant.
reactivity suppression. It is necessary to have applicability as the coolant for fast reactor well as sodium. So from the wide view point of design, operation and maintenance, it was verified that there is no critical problem of applicability by theoretical calculation and experiments. Sodium nanofluid is almost used equally as sodium. In particular, concerns are erosion of pipe by nanoparticle and influence of cold trap. Here, influence on these phenomena is described as follows. Erosion by nanoparticles is explained at first. The nanoparticle is very small size and gravity is also small. It is nearly equal to zero. The nanoparticle bonds strongly the surrounding sodium atoms. Hence, the nanoparticle does not move alone and nanoparticles are accompanied and move with sodium. So nanoparticle does not collide directly to pipe and the erosion by nanoparticle does not occur. It was verified that the erosion of pipe surface does not occur in high speed sodium nanofluid flow (10m/sec) which is the maximum speed assumed in plant. Next, the influence of sodium nanofluid on the cold trap is explained. Concerns are to be catch nanoparticles by mesh and, to become the core of oxide and to be catch nanoparticle with oxide. The size of nanoparticle is very smaller than that of mesh, so the nanoparticle does not catch alone. Considering the possibility to be the core of oxide, in order to become the core, temperature of nanoparticle has to be lower than that of liquid sodium. From the evaluation of melting temperature of sodium nanofluid melting temperature of sodium nanofluid is same as that of sodium. It is verified that temperature of only nanoparticle does not become lower than that of sodium, so nanoparticle does not become the core of precipitation. Also, the experiments simulating principle of cold trap were carried out, the reaction suppression of reaction heat of sodium nanofluid was evaluated before and after experiment. As a result, the reaction suppression after experiment is same as that before experiment. It is verified that the suspension behavior of nanoparticles in liquid sodium does not change. From these results, it is indicated that sodium nanofluid does not influence on cold trap. The applicability of sodium nanofluid was investigated from the wide view point of design, operation and maintenance. As results, it becomes clear there is no critical problem to practical use.
(3-2) Mitigation of requirements and restrictions for countermeasure equipment
It became clear that the combustion temperature of sodium fire and thermal damage to surrounding structure reduced by applying sodium nanofluid. From this effect, there is a possibility to simplify the countermeasures to sodium fire. Also, the damage propagation of heat transfer tube will be mitigate in sodium-water reaction. From these results, the safety of fast reactor enhances by using sodium nanofluid.
(3-3) Application concept for fast reactor
Applying sodium nanofluid that suppressed chemical reactivity, not only the safety enhancement of fast reactor as explained earlier but also it is a possibility to create new concept for fast reactor. Sodium nanofluid expands a freedom of fast reactor design.
(3-4) Possibility of expanding coverage of sodium nanofluid for fast reactor
From previous studies, chemical reactivity suppression of sodium nanofluid contributes the safety enhancement of fast reactor and it indicates there is a possibility to create new design concepts of fast reactor. Because chemical reactivity suppression of sodium nanofluid clearly confirmed, there is a possibility to apply to harsh accident conditions (high temperature and large amount of sodium leak). There is a possibility that the reactivity suppression of sodium nanofluid applies to not only the sodium fire and sodium-water reaction but also high speed corrosion and sodium-concrete reaction.
(4) Conclusion
The idea of the suppression of high chemical reactivity which is a weak point of liquid sodium used for coolant of fast reactor was created. The atomic interaction between nanoparticle and sodium atom is utilized by suspending nanoparticles in liquid sodium. It was understood theoretically the atomic bonding between nanoparticle and sodium and the charge transfer from the surrounding sodium atom to nanoparticle. And It was verified that the cohesive energy density increased by strengthening atomic bonding. It became clear that there is a possibility of chemical reactivity suppression due to change of physical property by stable suspension of nanoparticle in liquid sodium. Sodium nanofluid was actually produced and the atomic interaction was verified by its physical property. Furthermore the reaction suppression of sodium nanofluid was confirmed using the combustion test and sodium-water reaction test. By the suppression of chemical reactivity of sodium itself, it was found that a possibility of not only the enhancement to safety of fast reactor but also the creation of new concept of fast reactor.
(5) Acknowledgments
Present study is the result of “Development of safety enhancement of the fast reactor by using nanoparticle suspension sodium” entrusted to “Japan Atomic Energy Agency” by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
6. Reference
- K. Ara, K. Sugiyama, H. Kitagawa, M. Nagai and N. Yoshioka: “Study on Chemical Reactivity Control of Sodium by Suspended Nanoparticles I”, J. Nucl. Sci. Technol., Vol.47, No.12 pp.1165-1170 (2010).
- K. Ara, K. Sugiyama, H. Kitagawa, M. Nagai and N. Yoshioka: “Study on Chemical Reactivity Control of Sodium by Suspended Nanoparticles II”, J. Nucl. Sci. Technol., Vol.47, No.12 pp.1171-1181 (2010).
- J. Saito and K. Ara, “A study of atomic interaction between suspended nanoparticles and sodium atoms in liquid sodium”, Nuclear Engineering and Design, Vol.240, pp.2664-2673 (2010).
- J. Saito, N. Yoshioka, M. Nagai and K. Ara: “Study on Chemical Reactivity Suppression and Coolant Applicability of Sodium with Suspended Nanoparticles”, International Conference on FAST REACTORS AND RELATED FUEL CYCLES: Safe Technologies and Sustainable Scenarios FR13, 4-7 March, Paris, France (2013).
- K. Ara, J. Saito; “Characteristics of Liquid Sodium with Suspended Nanoparticles - Atomic Interaction and Fundamental Property –“, Proceedings of Nanofluids: Fundamentals and Applications II, August 15-19, Montreal, CANADA (2010).
- J. Saito, K. Ara; “Characteristics of Liquid Sodium with Suspended Nanoparticles - Reaction Property –“, Proceedings of Nanofluids: Fundamentals and Applications II, August 15-19, Montreal, CANADA (2010).
- S. K. Das, S. U. S. Choi, W. Yu and T. Pradeep: ”NANOFLUIDS SCIENCE AND TECHNOLOGY”, John Wiley & Sons, New York, ISBN9780470074732 (2007).
- J. Buongiorno, “The International Nanofluid Property Benchmark Exercise (INPBE): Results and Future Prospects”, Proceedings of Nanofluids: Fundamentals and Applications II, August 15-19, Montreal, CANADA (2010).
- E. V. Timofeeva, “Investigation of base fluid and temperature effects on heat transfer characteristics of SiC nanofluids”, Proceedings of Nanofluids: Fundamentals and Applications II, August 15-19, Montreal, CANADA (2010).
- G. Park, S. J. Kim, H. S. Park, M. H. Kim: “The effect of titanium nanoparticles on Na–water vapor reaction at105℃”, Nuclear Engineering and Design, Vol.293, pp.105-111 (2015).
- S. J. Kim, G. Park, M. H. Kim, H. S. Park, J. Baek: “A theoretical study of Ti nanoparticle effect on sodium water reaction: Using ab initio calculation”. Nuclear Engineering and Design, Vol.281, pp.15-21 (2015).
- A. Suzuki, P. Bonnaud, M. C. Williams, P. Selvam, N. Aoki, M. Miyano, A. Miyamoto, J. Saito and K. Ara, “Effect of the Titanium Nanoparticle on the Quantum Chemical Characterization of the Liquid Sodium Nanofluid”, J. Phys. Chem. B, Vol.120, No.14, pp 3527–3539 (2016), DOI: 10.1021/acs.jpcb.5b11461.
- A. Suzuki, K. Inaba, Y. Ishizawa, R. Miura, N. Hatakeyama, A. Miyamoto, J. Saito, K. Ara; “Theoretical Evaluation for The Stability of Liquid Sodium containing a Titanium Nanoparticle”, Proceedings of ICAPP 2015, Nice, France, ICAPP-15015 (2015).
- A. Suzuki, K. Inaba, Y. Ishizawa, R. Miura, N. Hatakeyama, A. Miyamoto, J. Saito, K. Ara; “QUANTUM CHEMICAL EVALUATION FOR THE STABILITY OF LIQUID SODIUM CONTAINING TITANIUM NANOPARTICLES”, Proceedings of the 23th International Conference on Nuclear Engineering (ICONE23), Chiba, Japan, May 17-21, ICONE23-1005 (2015).
- T. Takada, A. Yamaguchi, K. Fukuzawa and K. Matsubara; “Numerical Methodology of Sodium-Water Reaction with Multiphase Flow Analysis”, Nuclear Science and Engineering, Vol.150, pp.221-236 (2005).
- T. Itami, J. Saito and K. Ara, “The Promising Features of New Nano Liquid Metals—Liquid Sodium Containing Titanium Nanoparticles (LSnanop)”, Metals 2015, 5, 1212-1240; doi:10.3390/met5031212.
- M. Nishimura, K. Nagai, T. Onojima, J. Saito, K. Ara and K. Sugiyama: “The sodium oxidation reaction and suppression effect of sodium with suspended nanoparticles - Growth behavior of dendritic oxide during oxidation –“, J. Nucl. Sci. Technol., Vol.49, No.1 pp.71-77 (2012).
- H. Kanda, N. Yoshioka, K. Ara, J. Saito, K. Nagai, “Study on a suppression of sodium-water reaction in SFR by applying sodium with suspended nanoparticles”, Proceedings of ICAPP 2015, Nice, France, ICAPP-15248 (2015).
Japan Society of Maintenology (ejam@jsm.or.jp)